Insights
Our latest thinking on the issues that matter most in biostabilization science and techonology. Explore our featured insights.
2025
Stabilizing antibodies for room temperature storage – Evidence for improved laboratory operations and stability
Ambient Biosciences and B2S Life Sciences partner to demonstrate the successful stabilization of antibodies for storage at ambient conditions. See data showing preserved molecular integrity and functionality, with enhanced laboratory efficiency and sustainability.
2024
Ambient Biosciences Seeks Early Access Program Partners to Trial Room Temperature Stabilization Technology for Biomolecules and Reagents
Ambient Biosciences introduces the Early Access Program, offering partners exclusive access to capillary-assisted vitrification (CAV) technology. Designed to stabilize biomolecules at ambient temperatures, this groundbreaking solution reduces cold storage reliance, lowers costs, and extends shelf life.
JOURNAL OF PHARMACEUTICAL AND BIOMEDICAL ANALYSIS
2024
Capillary mediated vitrification is a novel technique that enables storage of antibody critical reagents at ambient temperature: Impact on binding, structure, and laboratory sustainability
Antibodies and antibody conjugates are essential components of life science research, but their inherent instability necessitates cold storage or lyophilization, posing logistical and sustainability challenges. Capillary-mediated vitrification (CMV) has shown promise as a tool for improving biomolecule stability. In this study, we assess the feasibility of shipping and storing CMV-stabilized antibody reagents at ambient temperature using a purified rabbit polyclonal as a model system. The cumulative data supports the use of Capillary-Mediated Vitrification as a viable alternative to frozen reagent storage, with the potential to significantly impact reagent stability, assay performance, laboratory operations, and sustainability initiatives.

2024
Room Temperature Stable PCR Reagents Preparation Using Capillary-Mediated Vitrification Process
This research explores how Capillary-Mediated Vitrification (CMV) effectively stabilizes PCR reagents, enabling their storage at room temperature without compromising performance. This demonstrates that PCR master mixes processed with CMV maintain similar amplification profiles compared to frozen controls, even after 84 days of storage at 37°C.

2024
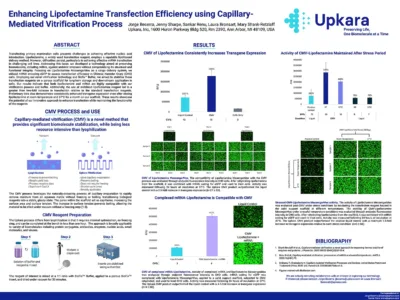
Enhancing Lipofectamine Transfection Efficiency using Capillary-Mediated Vitrification Process
Capillary-Mediated Vitrification (CMV) technology significantly improves the efficiency of Lipofectamine transfection. By stabilizing Lipofectamine and mRNA on a porous scaffold, CMV enhances transgene expression in CHO cells, achieving up to a 4.5-fold increase compared to liquid controls. This innovative approach is compatible with various biomolecules, offering a quick, non-freezing alternative to traditional methods, and maintains reagent activity even under stress conditions.
2024
Evaluation of Capillary-Mediated Vitrification (CMV) as an Alternative to Traditional Cold Temperature Storage for Kinase Assay Reagents
Capillary-Mediated Vitrification (CMV) enables ambient storage of kinase assay reagents, maintaining enzymatic activity without cold chain logistics. CMV-stabilized reagents perform comparably to those stored at -80°C, simplifying assay execution and reducing costs, making it an effective alternative for global distribution.

GENETIC ENGINEERING & BIOTECHNOLOGY NEWS
March 2024
Kick Cold-Chain Dependency: The Future of Antibody Storage Is Here
Current biomolecule storage methods rely on decades-old technologies, but today’s scientists need innovative solutions for state-of-the-art molecules and conjugates. Ambient Biosciences delivers ambient temperature-stable products that make science easier for you and your customers. This manufacturing-ready technology provides convenience, profitability and sustainability.

Journal of Immunological Methods
2023
Use of capillary-mediated vitrification to produce thermostable, single-use antibody conjugates as immunoassay reagents
This study explores the use of capillary-mediated vitrification (CMV) to enhance the storage and stability of antibody reagents. Traditional methods, which rely on freezing, often lead to material waste, freeze-thaw damage, and reagent contamination. By applying CMV, researchers were able to store antibodies in a thermostable, single-use format at elevated temperatures for up to three months. The study found that assays using CMV-stabilized reagents performed comparably to those using frozen controls, suggesting CMV could simplify workflows, reduce waste, and maintain reagent integrity.

PHARMSCI 360
2023
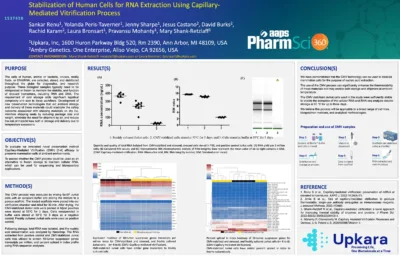
Stabilization of Human Cells for RNA Extraction Using Capillary-Mediated Vitrification Process
Ambient Bio (formerly Upkara), in collaboration with Promega, has applied Capillary-Mediated Vitrification (CMV) to enhance the thermal stability of luciferin, luciferase, and related reagents used in luminescence assays. This innovative process preserves reagent performance even after exposure to high temperatures, offering a reliable, ready-to-use alternative to traditional frozen reagents.
2023
Development of Thermal Stabile, Ready-to-Use Luminescence Reagents through the Application of Capillary-Mediated Vitrification
This study highlights the benefits of Capillary-Mediated Vitrification (CMV) in stabilizing luminescence reagents. Research shows that CMV effectively enhances the thermal stability of luciferase and luciferin, allowing ambient storage while maintaining performance, reducing costs, and extending shelf life.

PharmSci 360
2023
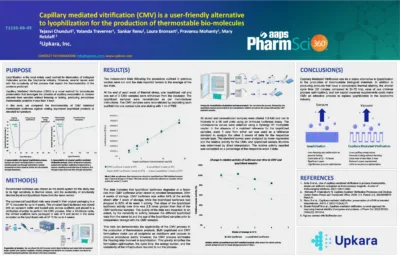
Capillary mediated vitrification (CMV) is a user-friendly alternative to lyophilization for the production of thermostable bio-molecules
This study presents a comparison between Capillary Mediated Vitrification (CMV) and traditional lyophilization for producing thermostable biomolecules. The findings indicate that CMV offers comparable thermal stability with significantly reduced cycle times, lower capital expenses, and simpler processing.
2023
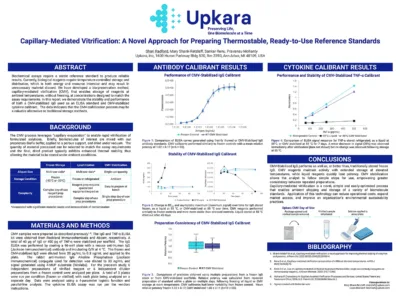
Capillary-Mediated Vitrification: A Novel Approach for Preparing Thermostable, Ready-to-Use Reference Standards
Capillary-Mediated Vitrification (CMV) stabilizes biomolecules at room temperature, eliminating the need for freezing. This method uses capillary evaporation and a proprietary buffer to create thermally stable, ready-to-use standards. Proven effective for IgG and cytokines in ELISA assays, CMV offers a simpler, more sustainable alternative to traditional storage, reducing costs and environmental impact.
2023
Improving the Stability and Performance of Bioassay Reagents using Capillary Mediated Vitrification
Capillary-Mediated Vitrification (CMV) enhances the stability and performance of various bioassay reagents, including antibodies, enzymes, and mRNA, without freezing. CMV-stabilized reagents perform comparably to frozen controls, even at elevated temperatures, offering a scalable and efficient alternative that reduces reliance on cold chain logistics and improves assay consistency.

2023
Improving the Stability and Performance of Assay Reagents Using Capillary-Mediated Vitrification
This study highlights the advantages of Capillary-Mediated Vitrification (CMV) in stabilizing and improving the performance of various bioassay reagents. This shows that CMV can effectively preserve antibodies, enzymes, and mRNA at elevated temperatures, maintaining their activity and reducing the need for cold storage.

BIOMATTERS
Spring 2023
Unchaining Innovation to Advance Health Equity
Imagine waiting for a lifesaving medication or vaccine only for it to arrive damaged after sitting in a hot shipping container for too long. Picture years of research and hard-earned grant funding being wasted because materials spoiled due to a delay on the tarmac. Though it isn’t always obvious, billions of people worldwide are affected by the inefficiencies of cold chain logistics.
Each year, the pharmaceutical industry alone spends approximately $18.6 billion on cold chain support to store and transport temperature-sensitive products. Losses related to cold chain failures add up to an additional $35 billion annually. Such costs are also challenging for universities, researchers, and reagent manufacturers.
But what if it was possible to stabilize fragile biomolecules without the use of freezing? Thanks to cutting-edge technology, it is.
Upkara is a diverse, forward-thinking biotechnology company headquartered in Ann Arbor, Michigan. It was founded by Pravansu Mohanty, Ph.D., as a spin-off of the Somnio think tank whose mission was to address global unmet needs. Upkara’s focus is biomolecule stabilization. Their novel technology, unlike the current stabilization technique of lyophilization or freeze drying, is rapid, cost-effective, and broadly applicable.

AMERICAN CHEMICAL SOCIETY
August 2022
Capillary-Mediated Vitrification: A Novel Approach for Preparing Thermostable, Ready-to-Use Reagents
Currently, biological reagents require temperature-controlled storage and distribution, which is both energy and resource intensive. Additionally, reagents are supplied at high concentrations and volumes resulting in significant material discard and complicated dilution schemes. We have developed a bio-preservation method, capillary-mediated vitrification (CMV), that enables storage of reagents at ambient temperatures and at concentrations designed to match the assay requirements. In this report, we demonstrate the stability and performance of CMV-stabilized reagents. CMV is a promising alternative to traditional biopreservation methods that significantly improves analytical workflow efficiencies and eliminates the need for cold storage.

THE AAPS JOURNAL
June 2022
Capillary-Mediated Vitrification: Preservation of mRNA at Elevated Temperatures
RNA is a fundamental tool for molecular and cellular biology research. The recent COVID-19 pandemic has proved it is also invaluable in vaccine development. However, the need for cold storage to maintain RNA integrity and the practical and economic burden associated with cold chain logistics highlight the need for new and improved preservation methods. We recently showed the use of capillary-mediated vitrification (CMV), as a tool for stabilizing temperature-sensitive enzymes. Here, we demonstrate the use of CMV as a method to preserve mRNA. The CMV process was performed by formulating a green fluorescent protein (GFP)-encoding mRNA with common excipients, applying the solution to a porous support, referred to as the scaffold, and drying the samples under vacuum for 30 min. The CMV preserved samples were stored at 55 °C for up to 100 days or 25 °C for 60 days and analyzed by electrophoresis and for transfection efficiency in a cell-based assay. The 55 °C-stressed mRNA exhibited comparable electrophoresis banding patterns and band intensity when compared to a frozen, liquid control. Additionally, the CMV stabilized mRNA maintained 97.5 ± 8.7% transfection efficiency after 77 days and 78.4 ± 3.9% after 100 days when stored 55 °C and analyzed using a cell-based assay in the CHO-K1 cell line. In contrast, a liquid control exhibited no bands on the electrophoresis gel and lost all transfection activity after being stored overnight at 55 °C. Likewise, after 60 days at 25 °C, the CMV-processed samples had full transfection activity while the activity of the liquid control was reduced to 40.1 ± 4.6%. In conclusion, CMV is a simple formulation method that significantly enhances the thermal stability of mRNA, requires minimal processing time, and could enable formulation of mRNA that can tolerate exposure to temperatures well above 25 °C during shipment and deployment in extreme environments.
JOURNAL OF PHARMACEUTICAL SCIENCES
February 2022
Capillary-Mediated Vitrification: A Novel Approach for Improving Thermal Stability of Enzymes and Proteins
Capillary-mediated vitrification (CMV) is a novel method for stabilizing biological molecules and complexes. CMV leverages capillary evaporation to enable rapid desiccation of aqueous solutions while avoiding both freezing and boiling. In the CMV process, an aqueous solution containing the biological material of interest and common excipients is applied to a solid, porous support, referred to as the scaffold, and desiccated under vacuum. The pores within the scaffold accelerate drying by increasing surface area while preventing boiling through the interaction of the vapor pressure, capillary forces, and viscous forces. The process, which can be completed in under an hour, produces an amorphous dried product with enhanced thermal stability. In this study, CMV is demonstrated using luciferase as a model system. Using a 30-minute drying time, residual moisture levels of <4% were achieved. CMV-stabilized luciferase maintained full activity when stored for up to 6 weeks at 25 °C and >70% activity after 6 weeks at temperatures up to 45 °C. The liquid formulated enzyme lost all activity after 1 day at 37 °C or 4 h at temperatures above 37 °C. The data presented in this report demonstrate that CMV is a promising alternative to traditional biopreservation methods.


