Shipping and storing temperature sensitive reagents and biomolecules often requires costly investments in the cold chain or time-consuming, burdensome, and outdated techniques such as lyophilization and cryopreservation.
WE HAVE A BETTER SOLUTION—
AND IT’S AMBIENT

Ambient Biosciences Stabilization
Ambient Biosciences Stabilization offers a superior alternative to lyophilization for producing shelf-stable reagents. Our gentle, freeze-free process stabilizes even the most sensitive biomolecules in a fraction of the time. Simply mix your reagent with our buffer, pipette the solution onto the scaffold, and place into the Ambient Bio chamber. It only takes an hour to achieve ambient temperature stabilization— or, let our experts handle it for you.
Wave goodbye to complicated lyophilization development programs and freezer maintenance. Instead, enjoy easy room-temperature storage that boosts profits and keeps customers happy.

Stable
Activity,
Guaranteed
Our patented process ensures your biomolecules retain their activity, even after years of storage at room temperature. Produce larger lot sizes with dramatically extended shelf lives, minimizing expired product waste.
Our patented process ensures your biomolecules retain their activity, even after years of storage at room temperature. Produce larger lot sizes with dramatically extended shelf lives, minimizing expired product waste.
We stabilize:
Antibodies & Conjugates:
ELISA, IHC, IF, FCM, FACS
Enzymes:
Luciferases, kinases, restriction enzymes, and PCR Kits
DNA/RNA:
Nucleic acids for gene transfer or specimen preservation
Small Molecules:
Substrates, buffers, chelators, and reducing agents
Cells:
Cells for downstream analytics or biorepositories
Others:
Master mixes and additional biomolecules not listed
The best part?
Ambient Biosciences’s stabilization process is easier to implement across biomolecule classes with minimal optimization and development compared with lyophilization. This means less time between product conception and product launch.
Extend Product Shelf Life
and Reduce Waste
Extend expiration dates from 6 months to over 2 years
Produce larger lot sizes with single-use reagent options
Eliminate risk of temperature excursions during shipment and freezer failure
Reduce material waste with master mix formulations
1. Sample Preparation
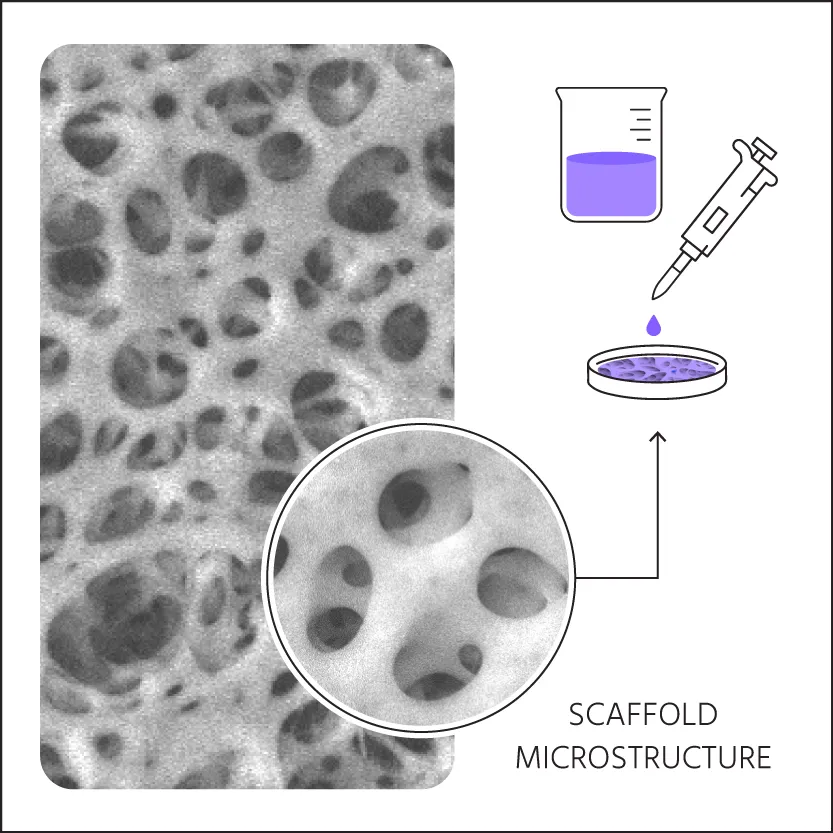
1. Sample Preparation
Biomolecules are prepared in a proprietary solution and loaded into a microporous scaffold.
Biomolecules are prepared in a proprietary solution and loaded into a microporous scaffold.
2. Capillary Action
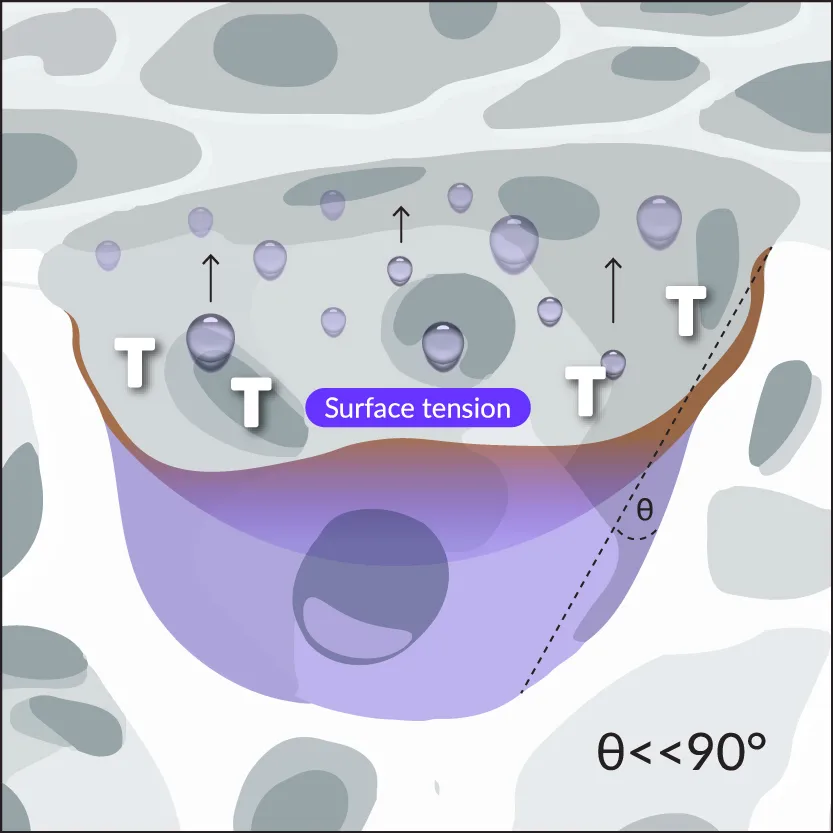
2. Capillary Action
The solution migrates into the scaffold via rapid capillary-assisted kinetics, resulting in high surface area-to-volume ratio and increased surface tension.
The solution migrates into the scaffold via rapid capillary-mediated kinetics, resulting in high surface area-to-volume ratio and increased surface tension.
3. Vitrification Process
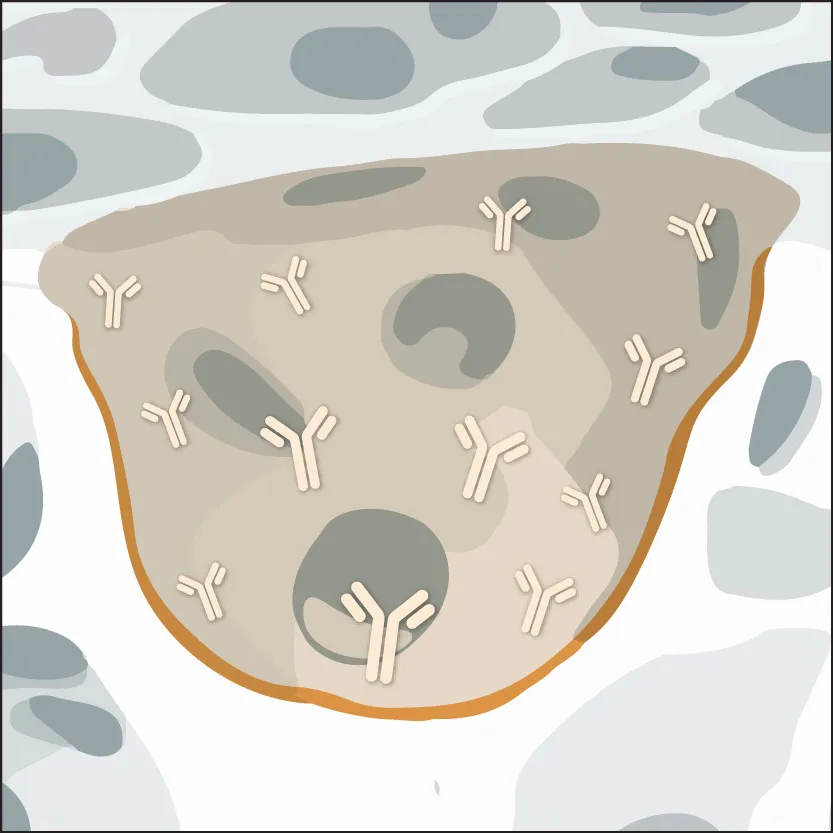
3. Vitrification Process
The solution undergoes rapid drying, generating stable biomolecules in a vitrified, glass-like state ready for ambient shipping and storage.
The solution undergoes rapid drying, generating stable biomolecules in a vitrified, glass-like state ready for ambient shipping and storage.
Ambient Biosciences Stabilization Phase Diagram
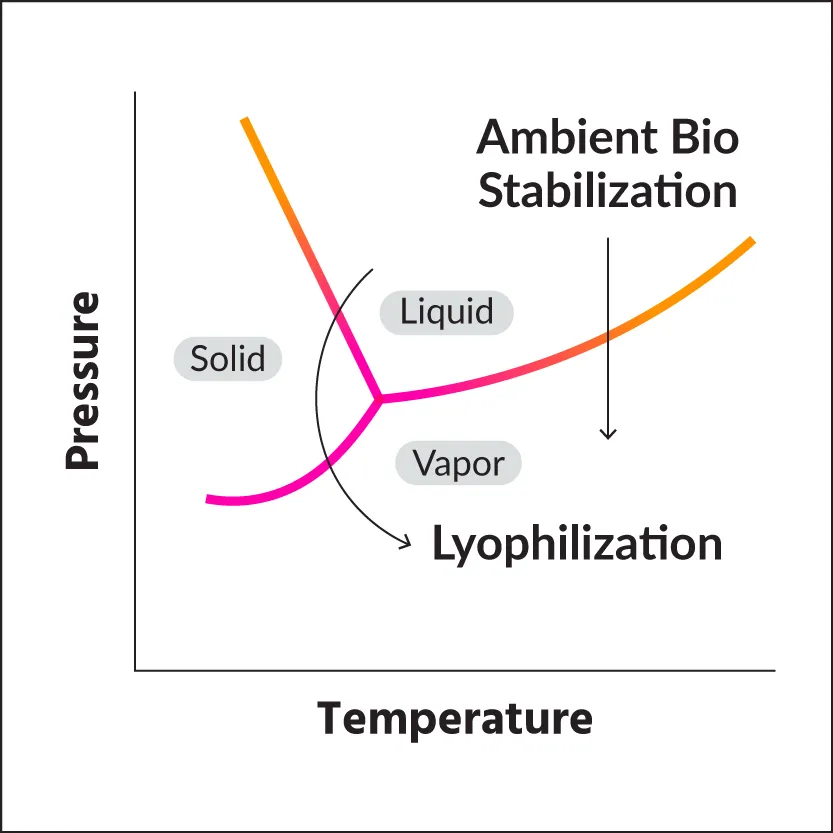
Ambient Biosciences Stabilization Phase Diagram
Ambient Biosciences’ stabilization process leverages the laws of physics to eliminate the freezing step required for effective lyophilization.

Simplify your workflows and streamline process time
Stabilize biomolecules with Ambient Bio in just a few steps— no complex lyophilization protocols or specialized training needed. Reconstituting is easy too, and you’ll recover more product in the long run.


Provide your customers with a better experience
- No more expensive cold shipping fees
- No need to dedicate precious lab space to freezers
- Easier and more consistent reconstitution
- Master mixes and user-friendly protocols
- Reduced errors from aliquoting or dilutions
- No more expensive cold shipping fees
- No need to dedicate precious lab space to freezers
- Easier and more consistent reconstitution
- Master mixes and user-friendly protocols
- Reduced errors from aliquoting or dilutions


We’re the stabilization experts that are easy to work with
With decades of experience, our team understands the need for reliable stabilization with hassle-free support. We provide a comprehensive technical consultation to help accelerate validation and get your products to market faster.
Unlike other providers, we’re flexible with small batches and offer rapid turnaround times. Our quality management system ensures reliable stabilization and product quality. We’re not just scientists – we’re committed partners dedicated to your success.

Our team collaborated with Ambient Biosciences to validate their ambient temperature storage technology for stabilization of monoclonal antibody biophysical properties and binding characteristics. The platform not only offers excellent performance compared to frozen storage, but provides cost and sustainability benefits as well. We believe this represents a potential breakthrough for ambient temperature storage, life cycle management, and global shipment of critical reagents, with broad application in biotherapeutics R&D.

In collaboration with Ambient Biosciences, we evaluated CMV-stabilized reagents for ease of use and functionality. While the convenience, cost savings, and sustainability aspects of ambiently shipped and stored reagents were major pluses, the technology really shined in multiplexed applications like flow cytometry. Although the format differed from standard liquid reagents, the method was easy-to-learn, and the pre-prepared reagents would likely result in fewer experimental errors and better data quality.

AssayQuant’s Phosphosens Technology relies heavily on cold chain distribution due to the temperature-sensitive components in our assay. Ambient Bio’s CMV technology enabled AssayQuant to develop an assay format that can be shipped at room temperature and simplifies the assay components onto a single scaffold. Ambient Bio’s technology has created new opportunities for the AssayQuant platform.

Storage and transportation of cellular specimens can be challenging due to the logistical complexities associated with the required cold chain and the high risk of temperature excursions degrading the materials. We were able to leverage Ambient Bio’s novel stabilization technology to successfully stabilize and store cell lysates for downstream analyses including RNA sequencing. The technology’s unique ability to stabilize highly complex matrices demonstrates its advantages for specimen shipping and bio repository applications.